Abstract
Introduction: Chimeric antigen receptor (CAR) T cell therapies have demonstrated robust and sustained clinical responses in several hematologic malignancies. Data suggest that achieving acceptable benefit:risk profiles depends on several factors, including the specificity of the antigen target and characteristics of the CAR itself, including on-target, off-tumor activity.To test the safety and efficacy of CAR T cells in relapsed and/or refractory multiple myeloma (RRMM), we have designed a second-generation CAR construct targeting B cell maturation antigen (BCMA) to redirect T cells to MM cells. BCMA is a member of the tumor necrosis factor superfamily that is expressed primarily by malignant myeloma cells, plasma cells, and some mature B cells. bb2121 consists of autologous T cells transduced with a lentiviral vector encoding a novel CAR incorporating an anti-BCMA scFv, a 4-1BB costimulatory motif and a CD3-zeta T cell activation domain.
Methods: CRB-401 (NCT02658929) is a multi-center phase 1 dose escalation trial of bb2121 in patients with RRMM who have received ≥ 3 prior regimens, including a proteasome inhibitor and an immunomodulatory agent, or are double-refractory, and have ≥ 50% BCMA expression on malignant cells. Peripheral blood mononuclear cells are collected via leukapheresis and shipped to a central facility for transduction, expansion, and release testing prior to being returned to the site for infusion. Patients undergo lymphodepletion with fludarabine (30 mg/m2) and cyclophosphamide (300 mg/m2) daily for 3 days then receive 1 infusion of bb2121. The study follows a standard 3+3 design with planned dose levels of 50, 150, 450, 800, and 1,200 x 106 CAR+ T cells. The primary outcome measure is incidence of adverse events (AEs), including dose-limiting toxicities (DLTs). Additional outcome measures were quality and duration of clinical response assessed according to the IMWG Uniform Response Criteria for Multiple Myeloma, evaluation of minimal residual disease (MRD), overall and progression-free survival, quantification of bb2121 in blood, and quantification of circulating soluble BCMA over time.
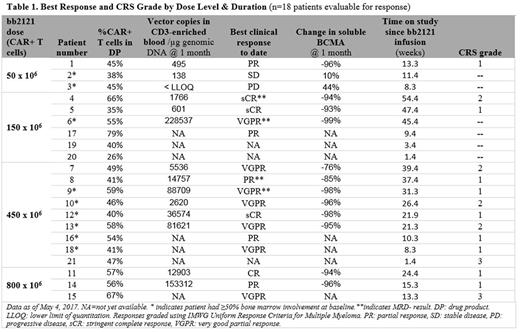
Results: Asof May 4, 2017, 21 patients (median 58 [37 to 74] years old) with a median of 5 (1 to 16) years since MM diagnosis, had been infused with bb2121, and 18 patients were evaluable for initial (1-month) clinical response. Patients had a median of 7 prior lines of therapy (range 3 to 14), all with prior autologous stem cell transplant; 67% had high-risk cytogenetics. Fifteen of 21 (71%) had prior exposure to, and 6 of 21 (29%) were refractory to 5 prior therapies (Bort/Len/Car/Pom/Dara). Median follow-up after bb2121 infusion was 15.4 weeks (range 1.4 to 54.4 weeks). As of data cut-off, no DLTs and no treatment-emergent Grade 3 or higher neurotoxicities similar to those reported in other CAR T clinical studies had been observed. Cytokine release syndrome (CRS), primarily Grade 1 or 2, was reported in 15 of 21 (71%) patients: 2 patients had Grade 3 CRS that resolved in 24 hours and 4 patients received tocilizumab, 1 with steroids, to manage CRS. CRS was more common in the higher dose groups but did not appear related to tumor burden. One death on study, due to cardiopulmonary arrest more than 4 months after bb2121 infusion in a patient with an extensive cardiac history, was observed while the patient was in sCR and was assessed as unrelated to bb2121. The overall response rate (ORR) was 89% and increased to 100% for patients treated with doses of 150 x 106 CAR+ T cells or higher. No patients treated with doses of 150 x 106 CAR+ T cells or higher had disease progression, with time since bb2121 between 8 and 54 weeks (Table 1). MRD negative results were obtained in all 4 patients evaluable for analysis. CAR+ T cell expansion has been demonstrated consistently and 3 of 5 patients evaluable for CAR+ cells at 6 months had detectable vector copies. A further 5 months of follow up on reported results and initial data from additional patients will be presented.
Conclusions: bb2121 shows promising efficacy at dose levels above 50 x 106 CAR+ T cells, with manageable CRS and no DLTs to date. ORR was 100% at these dose levels with 8 ongoing clinical responses at 6 months and 1 patient demonstrating a sustained response beyond one year. These initial data support the potential of CAR T therapy with bb2121 as a new treatment paradigm in RRMM.
CT.gov study NCT02658929, sponsored by bluebird bio and Celgene
Berdeja: Teva: Research Funding; Janssen: Research Funding; Novartis: Research Funding; Abbvie: Research Funding; Celgene: Research Funding; BMS: Research Funding; Takeda: Research Funding; Vivolux: Research Funding; Amgen: Research Funding; Constellation: Research Funding; Bluebird: Research Funding; Curis: Research Funding. Siegel: Celgene, Takeda, Amgen Inc, Novartis and BMS: Consultancy, Speakers Bureau; Merck: Consultancy. Jagannath: MMRF: Speakers Bureau; Bristol-Meyers Squibb: Consultancy; Merck: Consultancy; Celgene: Consultancy; Novartis: Consultancy; Medicom: Speakers Bureau. Turka: bluebird bio: Employment, Equity Ownership. Lam: bluebird bio: Employment, Equity Ownership. Hege: Celgene Corporation: Employment, Equity Ownership. Morgan: bluebird bio: Employment, Equity Ownership, Patents & Royalties. Quigley: bluebird bio: Employment, Equity Ownership, Patents & Royalties. Kochenderfer: Bluebird bio: Research Funding; N/A: Patents & Royalties: I have multiple patents in the CAR field.; Kite Pharma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal